Wet Age Related Macular Degeneration
Age Related Macular Degeneration is commonly divided into two types, dry and wet. Dry is a very slowly developing wear and tear of the retina at the back of the eye. Some people develop the wet form where new blood vessels grow under the centre of the retina. This can make central vision worse over a matter of weeks.
The blood vessels at the back of the eye are classified into 2 main groups by their appearance on the fluorescein angiogram. These are called classic and occult. Photodynamic therapy works best for the treatment of classic blood vessels.
Vascular Endothelial Growth Factor (VEGF)
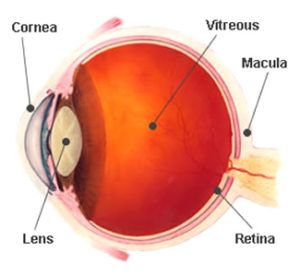
Vascular endothelial growth factor (VEGF) is a naturally occurring compound that is present throughout the body and the eye. It has many roles in maintaining the normal state of the retina and its blood vessels, which are only poorly understood. There are several different forms of VEGF.
One form in particular is greatly increased when new blood vessels form as part of Age Related Macular Degeneration. There are now available new compounds that are injected directly into the vitreous in the middle of the eye that block VEGF.
“I would like to say a huge thank you for your skill, expertise and professionalism in your approach to and care of myself.” Mrs U, Devizes
There are currently 5 drugs; Lucentis, Eylea, Avastin, Beovu and Vabysmo
Lucentis
Lucentis was developed from Avastin which is a drug licensed for the treatment of cancer of the bowel. It is part of an antibody that blocks all forms of VEGF including naturally occurring forms. The full blown antibody was thought to be too big to get across the retina to the underlying blood vessels and so the drug company developed Lucentis which has the same active end but is about ⅓ the size. Lucentis is given as an intravitreal injection every 4 weeks initially.
The main risk of Lucentis is that we do not know what the long-term effects to the eye may be of blocking all forms of VEGF rather than just the form that is greatly increased when new blood vessels grow. So far in all the drug tests carried out there have not been any long-term consequences.
The results of Lucentis trials suggest that over 2 years the chance of keeping good vision is in the region of 90 to 95%. Around 80% of patients gain some improvement in vision and between 20 and 30% of patients gain significant improvement in vision.
There are now generic versions of Lucentis available called Biosimilars.
Eylea
Eylea is a newer form of Anti-Vascular Endothelial Growth Factor Agent. It blocks more of the different types of VEGF as well as Placental Growth Factor. Trials have shown that it is probably as good as Lucentis. The trials have also been shown to suggest that patients could get away with fewer injections of Eylea.
Some patients who don’t respond very well to Lucentis may have more benefit with Eylea, particularly if there is swelling underneath the retina (pigment epithelial detachment).
Eylea 8 mg
There is now a more powerful version of Eylea called Eylea 8 mg. The original Eylea had 2 mg of the active drug aflibercept. The newer Eylea 8 mg is therefore 4 times as strong. Research studies suggested that it lasts longer than Eylea 2 mg and therefore that patients can get the same benefit with fewer injections.
Vabysmo
Vabysmo is another newer drug that blocks both VEGF and another cause of wet AMD, Angipoietin-2 (Ang-2). Research studies suggested that it is longer acting than Eylea 2 mg and that patients can get similar effects with fewer injections.
The loading course for Vabysmo is currently recommended to be 4 monthly injections rather than the 3 with Eylea.
Avastin
Avastin started to be used in its own right because doctors began to hear of the potential benefits of Lucentis but were unable to use it because it was still undergoing tests. They therefore tried using Avastin and found that in spite of its large size it did get across the retina and seemed to have good effects.
It has the advantage of being much cheaper than Lucentis, but all the safety work and testing that was done on Lucentis has not been done on Avastin.
There have been many thousands of injections of Avastin around the world with no significant problems reported. Avastin has a licence for use in colorectal cancer but not for injections into the eye. The American CATT study and the United Kingdom IVAN study comparing Avastin with Lucentis have published their 2 year results. These suggested that Avastin was similar to Lucentis but probably required more injections.
Beovu
Beovu was an Anti-Vascular Endothelial Growth Factor drug from the same family as Lucentis and Eylea. Clinical Research trials showed that it is as effective as Eylea but may be slightly longer acting so that fewer injections are needed overall.
In addition to the general risks of intravitreal injections about 4 in 100 patients can develop inflammation inside the eye and problems with the blood vessels at the back of the eye (retinal vasculitis).
Largely because of these side effects, Beovu is no longer used.